Machine learning. Artificial intelligence. Deep learning. Once purely seen as buzzwords, these concepts were eagerly discussed in theoretical conversations and lofty imaginings of what our future may hold. But now we find ourselves firmly in the data age, where artificial intelligence has become a critical tool in processing the staggering amount of information we produce. Machine learning techniques can be used to crunch the numbers and spot patterns where the human brain cannot.
Researchers are implementing their latest findings into software projects at an impressive rate. Often varied in nature and inspirationally innovative, these products enable a wide variety of practical applications of AI to help process data.
One sector where we are seeing greater demand for the use of artificial intelligence is in the healthcare arena, specifically when it comes to embedding technology in medical devices. We expect this trend to continue as new research emerges. Below, we look at some of the applications we have been involved in and share the advice we would offer to those looking to launch this type of innovative product.
TraCer Project
OCC collaborated with researchers from the University of Oxford, developing an application to support a portable ultrasound system for countries where experienced sonographers are in short supply. A portable probe and tablet device can measure the TransCerebellar diameter; a structure in the foetal brain which changes with gestational age. An application assists non-expert sonographers to record videos which are sent to the UK and interpreted using machine learning technology. Finding out the gestational age of the baby in places where this was not previously possible can have a hugely positive impact on obstetric care.
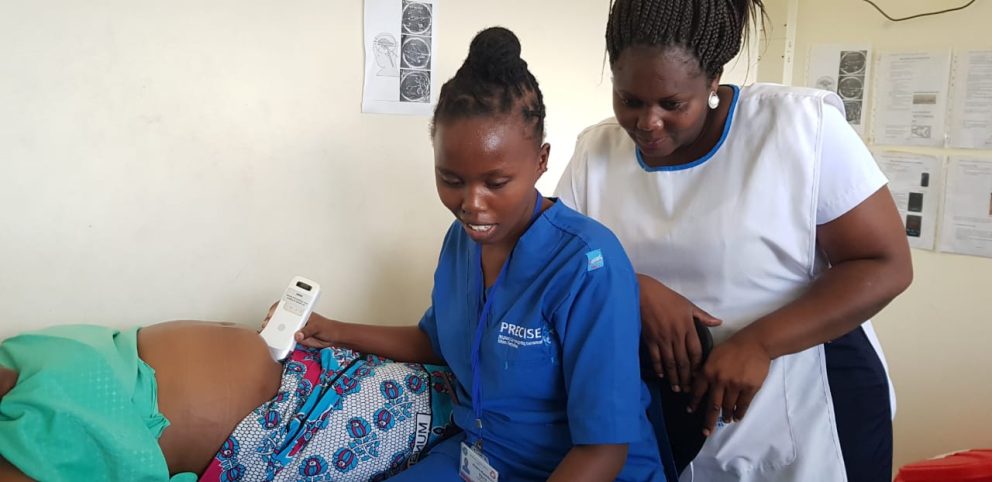
Park AI App
As part of this project, we assisted clinicians to monitor the emerging symptoms of Parkinson’s disease. Smartphone sensors are used by the app to detect and record the motions of users as they carry out exercises. This data is sent to the cloud, where artificial intelligence evaluates the severity score of the patient’s symptoms. Over time, the app will also track the progression of their symptoms. By analysing this data, clinicians can assess and plan improvements to the quality of life of the patients they treat. This research into Parkinson’s symptoms has been heralded as a ground-breaking approach to treatment in this field.
Taking an innovation to market: our viewpoint
It could be argued that an agile development approach is unsuited to medical or artificial intelligence projects, due to the perceived need to define the project upfront. However, this can be refuted by the fact that the agile approach is also characterised by flexibility, particularly in terms of development cycles and the opportunities for collaboration and feedback. This is why we employ this methodology when undertaking projects at OCC. Commencing with a thorough upfront discovery phase gives developers the chance to gain a complete picture of the customer’s vision. Despite the upfront analysis, the development plan is not set in stone, in fact the methodology allows for the brief to evolve during the project. Strong collaboration between clients, users and stakeholders is encouraged, and regular touchpoints and reviews are arranged. These give the opportunity for changes to be made based on the latest feedback. This approach is particularly useful when working with emerging research or cutting-edge products.
The importance of collaboration and sharing feedback in an agile way highlights the need for entrepreneurs to work with a software company who really understands the science behind the innovation. Developers who have a firm grip on the research will be best placed to develop a product in line with a client’s vision and to suggest meaningful improvements. This is applicable across all industries; customers should seek out a software company which will strive to understand the product they want to build.

Of course, there will be challenges on the route to market. Perhaps the biggest hurdle for medical device development companies is proving compliance with regulatory requirements, specifically when it comes to demonstrating product safety. Extensive documentation is required, but if consideration is given to the requirements from the project start, and if these are tackled methodically, this hurdle can be overcome. Particularly when working on medical projects, it is crucial to ensure diligent tracking of the product specification from day one. Of course, where an agile approach is used, the project specification may change as time goes on. This can present a challenge for teams that have produced detailed product specifications at the outset. At OCC, we improve and amend the product specification with each product iteration, so that everyone working on the project stays updated. The first iteration is prototyping, which involves the least amount of documentation. Although task tracking is mapped out, no details are included and only a minimal set of testing is undertaken. In the next iteration a market ready product is developed. As part of this step, detailed tasks will be added, along with risk assessments and testing. Regulatory finalisation forms the final iteration, where all task documentation is reviewed, additional testing is undertaken, and formal documentation is written up. Following these steps means that even though product development is rapid, at the end the client will still receive a technical file suitable for submission to a regulatory approval body.
One of the most exciting questions at the start of a project is the intended scale and scope of the innovation. Just how big does the client want the product to be? Is the objective to serve 10 requests a day or 10 million? Often when we commence a project, the artificial intelligence algorithm is a research program on a single computer that can process one job at a time. Once this algorithm is moved to the cloud it can cope with a vast number of concurrent users. This is why we advise working with a team that has experience delivering cloud services that are massively parallelisable by harnessing the latest in cloud technologies.
Future developments
Above we have highlighted just some of the examples of our collaboration with clients in this area and we have explored a few of the concerns and challenges they face. The field of machine learning and artificial intelligence is constantly evolving, and OCC wants to be involved at the cutting edge of the research. To this end, we are sponsoring an AI Fellowship in Machine Learning at Reuben College, University of Oxford. To find out more about this and our current projects using AI, you can see our AI webpage here: https://www.oxfordcc.co.uk/occ-artificial-intelligence/. If you would further information on working with us, please visit our website at https://www.oxfordcc.co.uk/ or email us at info@oxfordcc.co.uk.